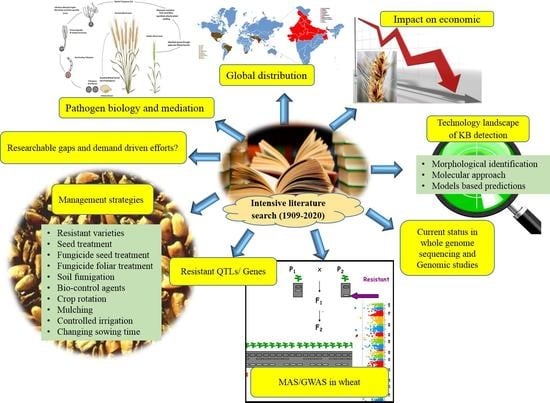
Centenary of Soil and Air Borne Wheat Karnal Bunt Disease Research: A Review
Abstract
:Simple Summary
Abstract
1. Introduction
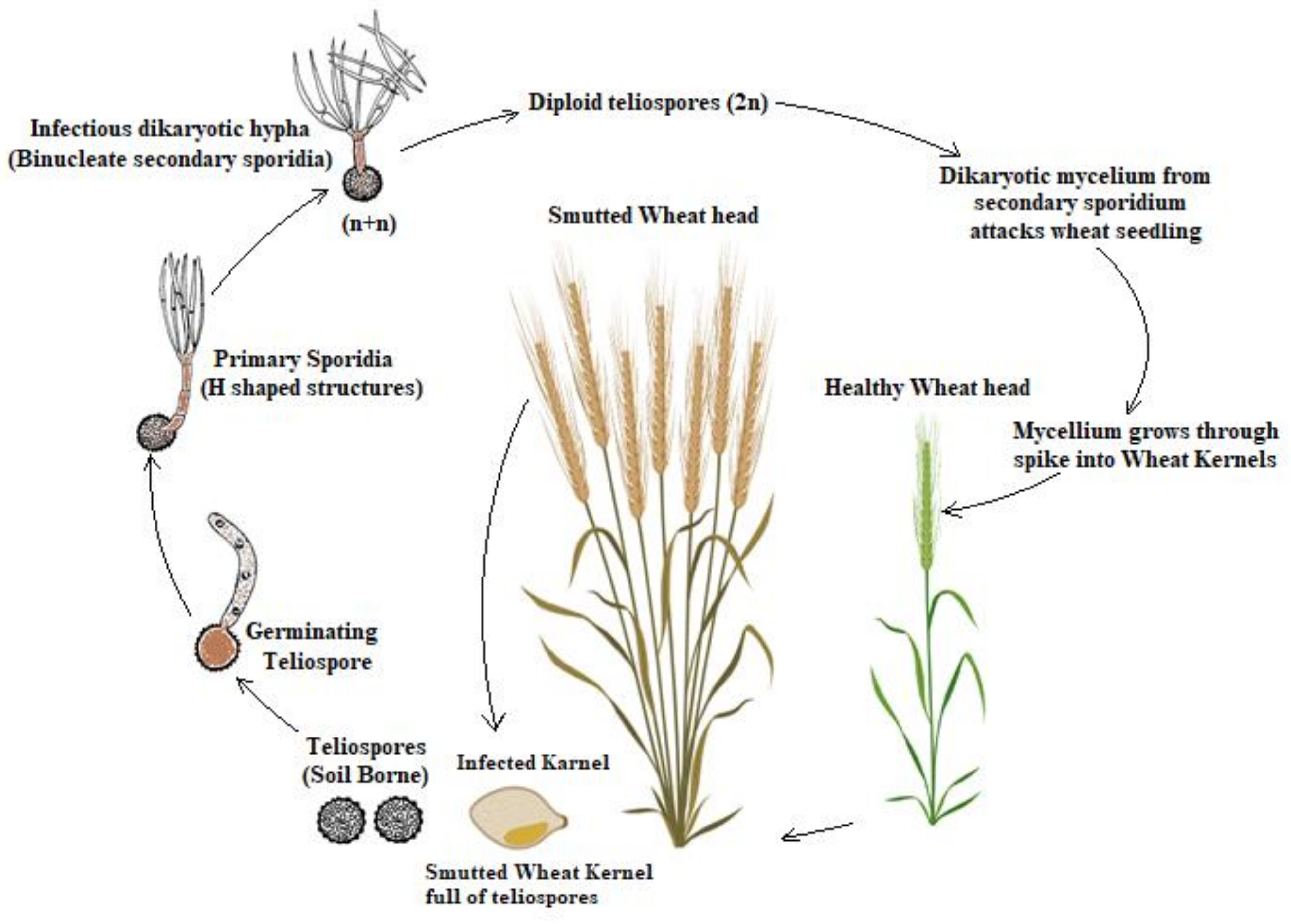
2. Pathogen Biology
3. Lifecycle
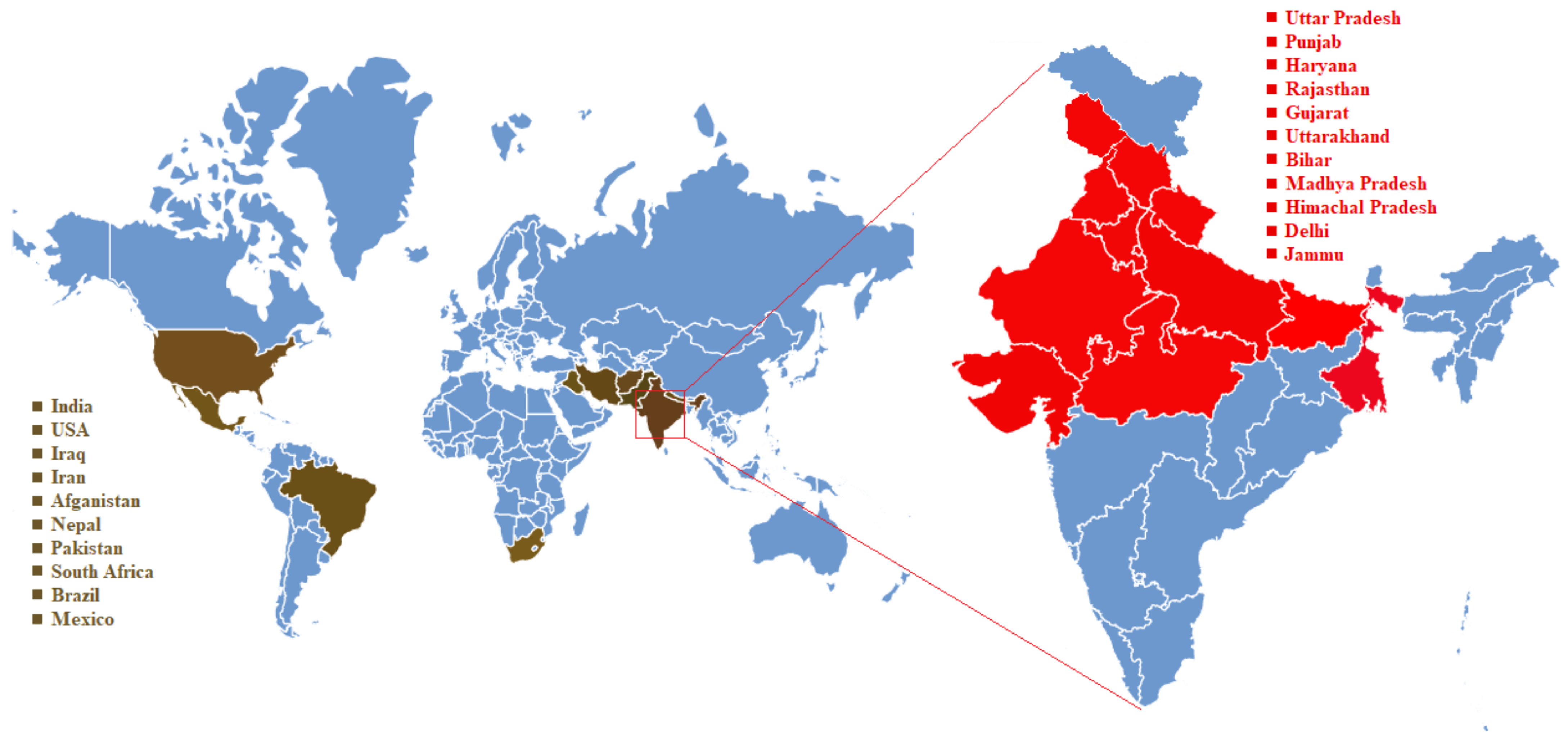
4. Karnal Bunt Disease Distribution over the Century
5. Impact of the Dynamics of Climate Change on Disease Development
6. The Disease Impact on Economic Extent
7. Technology Landscape of KB Detection
7.1. Morphological Identification
7.2. Molecular Approaches of Identification
7.2.1. Biophysical Techniques
7.2.2. Isozyme Based Techniques
7.2.3. DNA-Based Techniques
7.2.4. Immunological Techniques
7.2.5. ELISA
7.2.6. Tissue Imprinting
7.2.7. Immunofluorescence Assays
7.2.8. Immunodipsticks and Dot Blot
7.2.9. Agglutination Assay
7.2.10. Genetical Approach
7.3. Models for KB Disease Predictions
8. Revelation of Molecular Pathogenesis Mechanism by Multi-Omics Studies
9. KB Resistant Quantitative Trait Loci (QTLs)/Genes Mapped in Host Wheat
10. Status of MAS/GWAS in Wheat for KB Genome
11. Management Strategies for the Karnal Bunt
11.1. Use of Varieties Resistant to Karnal Bunt
11.2. Fungicide Seed Treatment
11.3. Fungicide Foliar Treatment
11.4. Soil Fumigation
11.5. Biocontrol Agents
11.6. Crop Rotation
11.7. Mulching
11.8. Controlled Irrigation
11.9. Changing Sowing Time
12. Major Researchable Gaps and Demand Driven Efforts in the Knowledge in KB Research
13. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Howard, A.; Howard, G.L.C. Wheat in India: I. Production, Varieties and Improvement; Thacker, Spink and Co.: Calcutta, India, 1909. [Google Scholar]
- Mitra, M. A New Bunt on Wheat in India. Ann. Appl. Biol. 1931, 18, 178–179. [Google Scholar] [CrossRef]
- Mundkur, B.B. Karnal bunt, an air-borne disease. Curr. Sci. 1943, 7, 230–231. [Google Scholar] [CrossRef]
- Vocke, G.; Allen, E.W.; Price, J.M. Economic Analysis of Ending the Issuance of Karnal Bunt Phytosanitary Wheat Export Certificates; Wheat Yearbook/WHS-2002; USDA Economic Research Service: Washington, DC, USA, 2002. [Google Scholar]
- Bedi, K.S.; Sikka, M.R.; Mundkar, B.R. Transmission of wheat bunt Neovossia indica (Mitra) Mundkar. Indian J. Phytopath. 1949, 2, 20–26. [Google Scholar]
- Joshi, A.K.; Mishra, B.; Chatrath, R.; Ortiz Ferrara, G.; Singh, R.P. Wheat improvement in India: Present status, emerging challenges and future prospects. Euphytica 2007, 157, 431–446. [Google Scholar] [CrossRef]
- Singh, D.V.; Gogoi, R. Karnal bunt of wheat (Triticum spp.)—A global scenario. Indian J. Agric. Sci. 2011, 81, 3–14. [Google Scholar]
- Juroszek, P.; Von Tiedemann, A. Climate change and potential future risks through wheat diseases: A review. Eur. J. Plant Pathol. 2012, 136, 21–33. [Google Scholar] [CrossRef] [Green Version]
- Haq, E.U.; Rattu, A.R.; Mirza, J.I. Prevalence of Karnal bunt of wheat in KPK (Pakistan). Int. J. Agric. Biol. 2002, 4, 150–152. [Google Scholar]
- Carris, L.M.; Castlebury, L.A.; Goates, B.J. Nonsystemic Bunt Fungi—Tilletia indica and T. horrida: A Review of History, Systematics, and Biology. Annu. Rev. Phytopathol. 2006, 44, 113–133. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.; Singh, U.; Kumar, J.; Garg, G. Application of molecular and immuno-diagnostic tools for detection, surveillance and quarantine regulation of Karnal bunt (Tilletia indica) of wheat. Food Agric. Immunol. 2008, 19, 293–311. [Google Scholar] [CrossRef] [Green Version]
- Tan, M.-K.; Ghalayini, A.; Sharma, I.; Yi, J.; Shivas, R.; Priest, M.; Wright, D. A one-tube fluorescent assay for the quarantine detection and identification of Tilletia indicaand other grass bunts in wheat. Australas. Plant Pathol. 2009, 38, 101–109. [Google Scholar] [CrossRef]
- Ullah, H.M.Z.; Haque, M.I.; Rauf, C.A.; Akhtar, L.H.; Munir, M. Comparative virulence in isolates of T. indica and host resistance against Karnal bunt of wheat. J. Anim. Plant Sci. 2012, 22, 467–472. [Google Scholar] [CrossRef]
- Fisher, G.W. Manual of the North American Smut Fungi; Ronald Press Co.: New York, NY, USA, 1953; p. 343. [Google Scholar]
- Jones, D.R. Towards a more reasoned assessment of the threat to wheat crops from Tilletia indica, the cause of Karnal bunt disease. Eur. J. Plant Pathol. 2008, 123, 247–259. [Google Scholar] [CrossRef]
- Singh, S.; Singh, M.; Taj, G.; Gupta, S.; Kumar, A. Development of Surface Plasmon Resonance (SPR) Based Immuno-Sensing System for Detection of Fungal Teliospores of Karnal Bunt (Tilletia indica), a Quarantined Disease of Wheat. J. Biosens. Bioelectron. 2012, 3, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Muhammad, A.; Muhammad, R.; Muhammad, S.; Aftab, B.; Muhammad, I. Response of some commercial cultivars and advanced lines of wheat against Karnal bunt of wheat and its management through chemicals. Int. J. Plant Res. 2013, 3, 47–51. [Google Scholar] [CrossRef]
- Parveen, S.; Saharan, M.S.; Verma, A.; Sharma, I. Comparative analysis of RAPD and ISSR marker assays for detecting genetic polymorphism in T. indica. Eur. J. Plant Pathol. 2013, 3, 380–387. [Google Scholar]
- Fuentes-Dávila, G.; Durán, R. Tilletia indica: Cytology and teliospore formation in vitro and in immature kernels. Can. J. Bot. 1986, 64, 1712–1719. [Google Scholar] [CrossRef]
- Bonde, M.R.; Berner, D.K.; Nester, S.E.; Peterson, G.L.; Olsen, M.W.; Cunfer, B.M.; Sim, T. Survival of Tilletia indica Teliospores in Different Soils. Plant Dis. 2004, 88, 316–324. [Google Scholar] [CrossRef] [Green Version]
- Joshi, L.M.; Singh, D.V.; Srivastava, K.D.; Wilcoxson, R.D. Karnal bunt: A minor disease that is now a threat to wheat. Bot. Rev. 1983, 49, 309–330. [Google Scholar] [CrossRef]
- CABI. Tilleta indica. In Distribution Maps of Plant Diseases; International Mycological Institute: Wallingford, UK, 1996; Volume 5, p. 173. [Google Scholar]
- Singh, D.V. Karnal bunt of wheat: A global perspective. Indian Phytopathol. 2005, 58, 1–9. [Google Scholar]
- Mundkur, B. A second contribution towards a knowledge of Indian ustilaginales. Trans. Br. Mycol. Soc. 1940, 24, 312–336. [Google Scholar] [CrossRef]
- Dastur, J.F. Report of the imperial mycologist. In Science Reports of the Agricultural Research Institute; Agricultural Research Institute: New Delhi, India, 1946; pp. 66–72. [Google Scholar]
- Mustafa, F.H. A list of common plant diseases in Iraq. Bull. Minist. Agric. Iraq 1965, 111, 1–16. [Google Scholar]
- Singh, D.V.; Agarwal, R.; Shrestha, J.K.; Thrapa, B.R.; Dubin, J.J. First Report of Tilletia indica on Wheat in Nepal. Plant Dis. 1989, 73, 273. [Google Scholar] [CrossRef]
- Torarbi, M.; Mardoukhi, V.; Jalaiani, N. First report on the occurrence of partial bunt on wheat in the southern parts of Iran. Seed Plant 1996, 12, 8–9. [Google Scholar]
- Nath, R.; Lambat, A.K.; Mukewar, P.M.; Rani, I. Interceptions of pathogenic fungi on imported seed and planting material. Indian Phytopathol. 1981, 34, 282–286. [Google Scholar]
- Diekmann, M. Monitoring the presence of Karnal bunt (Tilletia indica) in germplasm exchange at ICARDA. Phytopathol. Mediterr. 1987, 26, 59–60. [Google Scholar]
- Warham, E.J. Karnai bunt disease of wheat: A literature review. Trop. Pest Manag. 1986, 32, 229–242. [Google Scholar] [CrossRef]
- Rush, C.M.; Stein, J.M.; Bowden, R.L.; Riemenschneider, R.; Boratynski, T.; Royer, M.H. Status of Karnal Bunt of Wheat in the United States 1996 to 2004. Plant Dis. 2005, 89, 212–223. [Google Scholar] [CrossRef] [Green Version]
- DaLuz, W.C.; Mendes, M.A.S.; Fereira, M.A.S.V.; Urben, A.F. Tilletia indica on wheat in the southern part of Rio Grande do Sul and means for its eradication. Fitopatol. Bras. 1993, 18, 329. [Google Scholar]
- Crous, P.W.; Van Jaarsveld, A.B.; Castlebury, L.A.; Carris, L.M.; Frederick, R.D.; Pretorius, Z.A. Karnal Bunt of Wheat Newly Reported from the African Continent. Plant Dis. 2001, 85, 561. [Google Scholar] [CrossRef]
- Joshi, L.M.; Singh, D.V.; Srivastava, K.D. Problems and Progress of Wheat Pathology in South Asia; Malhotra Publishing House: New Delhi, India, 1986; p. 401. [Google Scholar]
- Singh, D.V.; Srivastava, K.D.; Goel, L.B.; Joshi, L.M.; Gupta, R.S. Incidence of Karnal bunt in Northwestern India during 1974–75. Indian Phytopathol. 1977, 30, 431–432. [Google Scholar]
- Singh, D.V.; Srivastava, K.D.; Joshi, L.M. Present status of Karnal bunt of wheat in relation to its distribution and varietal susceptibility. Indian Phytopathol. 1985, 38, 507–515. [Google Scholar]
- Sharma, A.K.; Kumar, J.; Nagarajan, S.S. Worldwide movement of smuts and bunts. In Bunts and Smuts of Wheat: An International Symposium; Malik, V.S., Mathre, D.E., Eds.; North American Plant Protection Organization (NAPPO): Ottawa, ON, Canada, 1998; pp. 129–136. [Google Scholar]
- ICAR-IIWBR Annual Report 2018–2019. Available online: https://iiwbr.icar.gov.in/annual-report/ (accessed on 20 June 2020).
- Jhorar, O.P.; Mavi, H.S.; Sharma, I.; Mahi, G.S.; Mathauda, S.S.; Singh, G. A biometeorological model for forecasting Karnal bunt disease of wheat. Plant Dis. Res. 1992, 7, 204–209. [Google Scholar]
- Chakraborty, S.; Tiedemann, A.; Teng, P. Climate change: Potential impact on plant diseases. Environ. Pollut. 2000, 108, 317–326. [Google Scholar] [CrossRef]
- Munjal, R.L. Studies of Karnal bunt (Neovossia indica) of wheat in northern India during 1968–1969 and 1969–1970. Indian J. Mycol. Plant Pathol. 1976, 5, 185–187. [Google Scholar]
- Singh, A. Epidemiology and Management of Karnal Bunt Disease of Wheat; Research Bulletin No. 127; Directorate of Experiment Station, G.B. Pant University of Agriculture & Technology: Pantnagar, India, 1994; p. 167. [Google Scholar]
- Hassan, S.F. Wheat diseases and their relevance to improvement and production in Pakistan. In Proceedings of the Fourth, FAO/Rockfeller Foundation Wheat Seminar, Tehran, Iran, 21 May 1973; pp. 84–86. [Google Scholar]
- Fuentes-Davila, G. Karnal bunt of wheat. In Bunts and Smuts of Wheat: An International Symposium; Malik, V.S., Mathre, D.E., Eds.; North American Plant Protection Organization (NAPPO): Ottawa, ON, Canada, 1998; p. 445. [Google Scholar]
- Tan, M.-K.; Brennan, J.P.; Wright, D.; Murray, G.M. A review of the methodology to detect and identify Karnal bunt—A serious biosecurity threat. Australas. Plant Pathol. 2012, 42, 95–102. [Google Scholar] [CrossRef]
- Bertolini, E.; Olmos, A.; Caruso, P.; Llop, P.; Cambra, M. Innovative tools for detection of plant pathogenic viruses and bacteria. Int. Microbiol. 2003, 6, 233–243. [Google Scholar] [CrossRef]
- Gupta, V.; Kumar, A.; Singh, A.; Garg, G.K. Immunodetection of teliospores of Karnal bunt (Tilletia indica) of wheat of viz., fluorescent staining test. Plant Cell Biotechnol. Mol. Biol. 2001, 1, 81–86. [Google Scholar]
- Micales, J.A.; Bonde, M.R.; Peterson, G.L. The use of isozyme analysis in fungal taxonomy and genetics. Mycataxon 1986, 27, 405–449. [Google Scholar]
- Bonde, M.R.; Pesterson, G.L.; Dowler, W.M.; May, B. Comparison of Tilletia indica isolates from India and Mexico by isome analysis. Plant Dis. 1985, 75, 1309–1316. [Google Scholar] [CrossRef]
- Bonde, M.R.; Tooley, P.W.; Schaasd, N.W. A PCR technique for accurate identification of teliospores of the Karnal bunt pathogen, Tilletia indica. In Proceedings of the 2nd ISTA-PDC (Plant Disease Committee) Symposium-Seed Health Testing towards the 21st Century, Cambridge, UK, 5–8 August 1997. [Google Scholar]
- Sikora, K.; Smedley, H.M. Monoclonal Antibodies; Blackwell Science: Oxford, UK, 1984. [Google Scholar]
- Varshney, G.K. Characterization of Genetic Races of Karnal Bunt (Tilletia indica) of Wheat by Immunobinding Assay Procedure. Master’s Thesis, G.B. Pant University of Agricultural & Technology, Pantnagar, India, 1999. [Google Scholar]
- Rai, G.; Kumar, A.; Garg, G.K.; Singh, A.; Lakhchaura, B.D. Development of microtitre ELISAs for detection and quantitation of mycelial antigens of Karnal bunt (Tilletia indica). Indian J. Agric. Biochem. 1998, 11, 53–55. [Google Scholar]
- Lin, N.S.; Hsu, Y.H.; Hsu, H.T. Immunological Detection of Plant Viruses and a Mycoplasmalike Membranes by Direct Tissue Blotting on Nitrocellulose Membranes. Phytopathology 1990, 80, 824–828. [Google Scholar] [CrossRef]
- Makkouk, K.M.; Hsu, H.T.; Kumari, S.G. Detection of Three Plant Viruses by Dot-Blot and Tissue-Blot Immunoassays Using Chemiluminescent and Chromogenic Substrates. J. Phytopathol. 1993, 139, 97–102. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, A.; Garg, G.K. Development of Seed Immunoblot Binding Assay for Detection of Karnal bunt (Tilletia indica) of Wheat. J. Plant Biochem. Biotechnol. 1998, 7, 119–120. [Google Scholar] [CrossRef]
- Kesari, R.; Kumar, A. Design and optimization of novel immuno-dipstick test for detection of teliospores of Karnal Bunt (Tilletia indica) of wheat. IJBT 2003, 2, 603–606. [Google Scholar]
- Kesari, R.; Mishra, D.P.; Garg, G.K.; Kumar, A. Suitability of dyed latex bead agglutination test for immunodiagnosis of Karnal bunt (Tilletia indica) teliospores in a single seed of wheat. Food Agric. Immunol. 2005, 16, 73–81. [Google Scholar] [CrossRef]
- Nagarajan, S. Epidemiology of Karnal Bunt of Wheat Incited by Neovossia Indica and an Attempt to Develop a Disease Predictive System; Wheat Special Report No. 4; CIMMYT: El Batán, Mexico, 1991; p. 69. [Google Scholar]
- Diekmann, M. Epidemiology and Geophytopathology of Selected Seed-Borne Diseases; International Center for Agricultural Research in the Dry Areas (ICARDA): Aleppo, Syria, 1993; p. 77. [Google Scholar]
- Bishnoi, M.R.K. Takayasu arteritis: A comprehensive review of literature. Int. J. Res. Med. Sci. 2020, 8, 3418–3425. [Google Scholar] [CrossRef]
- Gurjar, M.S.; Aggarwal, R.; Jogawat, A.; Kulshreshtha, D.; Sharma, S.; Solanke, A.U.; Dubey, H.; Jain, R.K. De novo genome sequencing and secretome analysis of Tilletia indica inciting Karnal bunt of wheat provides pathogenesis-related genes. 3 Biotech 2019, 9, 1–11. [Google Scholar] [CrossRef]
- Pandey, V.; Singh, M.; Pandey, D.; Marla, S.; Kumar, A. Secretome analysis identifies potential path-ogenicity/virulence factors of Tilletia indica, a quarantined fungal pathogen inciting Karnal bunt disease in wheat. Proteomics 2018, 18, 1700473. [Google Scholar] [CrossRef]
- Singh, J.; Aggarwal, R.; Gurjar, M.S.; Sharma, S.; Jain, S.; Saharan, M.S. Identification and expression analysis of pathogenicity-related genes in Tilletia indica inciting Karnal bunt of wheat. Australas. Plant Pathol. 2020, 49, 393–402. [Google Scholar] [CrossRef]
- Sharma, P.; Tiwari, R.; Saharan, M.S.; Sharma, I.; Kumar, J.; Mishra, S.; Muthusamy, S.K.; Gupta, R.K.; Jaiswal, S.; Iquebal, M.A.; et al. Draft Genome Sequence of Two Monosporidial Lines of the Karnal Bunt Fungus Tilletia indica Mitra (PSWKBGH-1 and PSWKBGH-2). Genome Announc. 2016, 4, 00928–16. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.; Pandey, V.; Singh, M.; Pandey, D.; Saharan, M.S.; Marla, S.S. Draft genome sequence of Karnal bunt pathogen (Tilletia indica) of wheat provides insights into the pathogenic mechanisms of quarantined fungus. PLoS ONE 2017, 12, e0171323. [Google Scholar] [CrossRef]
- Mishra, P.; Maurya, R.; Gupta, V.K.; Ramteke, P.W.; Marla, S.S.; Kumar, A. Comparative genomic analysis of monosporidial and monoteliosporic cultures for unraveling the complexity of molecular pathogenesis of Tilletia indica pathogen of wheat. Sci. Rep. 2019, 9, 8185. [Google Scholar] [CrossRef]
- Nguyen, H.D.T.; Sultana, T.; Kesanakurti, P.; Hambleton, S. Genome sequencing and comparison of five Tilletia species to identify candidate genes for the detection of regulated species infecting wheat. IMA Fungus 2019, 10, 1–17. [Google Scholar] [CrossRef]
- Singh, J.; Aggarwal, R.; Gurjar, M.; Sharma, S.; Saharan, M. Characterisation of Isolates of Tilletia indica Inciting Karnal Bunt of Wheat (Triticum aestivum L.). Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 2080–2087. [Google Scholar] [CrossRef]
- Indu-Sharma, G.; Nanda, S.; Chand, K.; Sohu, V.S. Status of Karnal bunt (Tilletia indica) resistance in bread wheat (Triticum aestivum). Indian J. Agric. Sci. 2001, 71, 794–796. [Google Scholar]
- Kaur, S.; Nanda, G.S. Identifying differential susceptibility to Karnal bunt in bread wheat. Crop Improv. 2002, 29, 164–168. [Google Scholar]
- Singh, D.P.; Sinha, V.C.; Goel, L.B.; Sharma, I.; Aujla, S.S.; Karwasra, S.S.; Tewari, A.N. Confirmed sources of resistance to Karnal bunt in wheat in India. Plant Dis. Res. 2003, 18, 37–38. [Google Scholar]
- Brown-Guedira, G.L.; Grewal, T.S.; Dhaliwal, H.S.; Nelson, J.C.; Singh, H.; Gill, B.S. Mapping of a resistance gene effective against Karnal bunt pathogen of wheat. Theor. Appl. Genet. 2003, 106, 287–292. [Google Scholar] [CrossRef]
- Sharma, I.; Bains, N.S.; Nanda, G.S. Inheritance of Karnal bunt-free trait in bread wheat. Plant Breed. 2004, 123, 96–97. [Google Scholar] [CrossRef]
- Singh, S.; Sharma, I.; Sehgal, S.K.; Bains, N.S.; Guo, Z.; Nelson, J.C.; Bowden, R.L. Molecular mapping of QTLs for Karnal bunt resistance in two recombinant inbred populations of bread wheat. Theor. Appl. Genet. 2007, 116, 147–154. [Google Scholar] [CrossRef]
- Hernandez, M.V.; Crossa, J.; Singh, P.K.; Bains, N.S.; Singh, K.; Sharma, I. Multi-Trait and Multi-Environment QTL Analyses for Resistance to Wheat Diseases. PLoS ONE 2012, 7, e38008. [Google Scholar] [CrossRef] [Green Version]
- Brar, G.S.; Fuentes-Dávila, G.; He, X.; Sansaloni, C.P.; Singh, R.P.; Singh, P.K. Genetic Mapping of Resistance in Hexaploid Wheat for a Quarantine Disease: Karnal Bunt. Front. Plant Sci. 2018, 9, 1497. [Google Scholar] [CrossRef] [PubMed]
- Emebiri, L.; Singh, S.; Tan, M.-K.; Singh, P.K.; Fuentes-Dávila, G.; Ogbonnaya, F. Unravelling the Complex Genetics of Karnal Bunt (Tilletia indica) Resistance in Common Wheat (Triticum aestivum) by Genetic Linkage and Genome-Wide Association Analyses. G3 Genes Genomes Genet. 2019, 9, 1437–1447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, V.; He, X.; Kumar, N.; Fuentes-Davila, G.; Sharma, R.K.; Dreisigacker, S.; Juliana, P.; Ataei, N.; Singh, P.K. Genome Wide Association Study of Karnal Bunt Resistance in a Wheat Germplasm Collection from Afghanistan. Int. J. Mol. Sci. 2019, 20, 3124. [Google Scholar] [CrossRef] [Green Version]
- Singh, S.; Sehgal, D.; Kumar, S.; Arif, M.A.R.; Vikram, P.; Sansaloni, C.P.; Fuentes-Dávila, G.; Ortiz, C. GWAS revealed a novel resistance locus on chromosome 4D for the quarantine disease Karnal bunt in diverse wheat pre-breeding germplasm. Sci. Rep. 2020, 10, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Singh, D. Integrated management of wheat Karnal bunt. Int. J. Pest Manag. 1993, 39, 431–434. [Google Scholar] [CrossRef]
- Kumar, A.; Gupta, A.; Atwal, S.; Maheshwari, V.; Singh, C. Post Harvest Management of Karnal Bunt, A Quarantine Disease in Wheat. Plant Pathol. J. 2015, 14, 23–30. [Google Scholar] [CrossRef]
- Jafari, H.; Torabi, M.; Alizadeh, A. Evaluation of resistance of some advanced cultivars/lines of wheat to T. indica, the causal organism of Karnal bunt. Seed Plant 2000, 15, 296–312. [Google Scholar]
- El-Naimi, M.; Toubia-Rahme, H.; Mamluk, O.F. Organic Seed-Treatment as a Substitute for Chemical Seed-Treatment to Control Common Bunt of Wheat. Eur. J. Plant Pathol. 2000, 106, 433–437. [Google Scholar] [CrossRef]
- Sharma, B.K.; Basandrai, A.K. Effectiveness of some fungicides and bio-control agents for the management of Karnal bunt of wheat. Ind. J. Mycol. Plant Pathol. 2000, 30, 76–78. [Google Scholar]
- Sharma, R.; Kumar, R. Karnal bunt disease of wheat study from Jhunjhunu, Rajasthan. Int. J. Adv. Res. Ideas Innov. Technol. 2017, 3, 834–835. [Google Scholar]
- Porter, P.M.; Huggins, D.R.; Perillo, C.A.; Quiring, S.R.; Crookston, R.K. Organic and Other Management Strategies with Two- and Four-Year Crop Rotations in Minnesota. Agron. J. 2003, 95, 233–244. [Google Scholar] [CrossRef]
- Brooks, S.A.; Anders, M.M.; Yeater, K.M. Influences from Long-Term Crop Rotation, Soil Tillage, and Fertility on the Severity of Rice Grain Smuts. Plant Dis. 2011, 95, 990–996. [Google Scholar] [CrossRef]
- Wright, D.; Murray, G.; Brennan, J. Draft national contingency plan for Karnal bunt of wheat. Part I background and importance. Plant Health Aust. 2006, 15, 1–79. [Google Scholar]
- Stansbury, C.D.; Pretorius, Z.A. Modelling the potential distribution of Karnal bunt of wheat in South Africa. S. Afr. J. Plant Soil 2001, 18, 159–168. [Google Scholar] [CrossRef]
| Tilletia Species | Teliospore Size (µm) | Ornamentation Color | Surface View | Profile |
|---|---|---|---|---|
| T. indica | 28–54 | Densely tuberculate to narrowly cerebriform | Conical to truncate | Dark reddish brown to black, opaque |
| T. inolens | 31–41 | Coarse polygonal scales, made up of many fine spines | Coarse, cylindrical, flared and broken at tips | Dark golden yellow to dark chocolate brown |
| T. cathcartae | 30–40 | Densely verruculose to tuberculate | Cylindrical, truncate | Golden brown |
| T. eragrostidis | 28–37 | Coarse polygonal scales | Coarse truncate projections | Light to dark reddish brown |
| T. walkeri | 23–45 | Coarsely cerebriform to coralloid scales | Conical to truncate | Pale yellow to dark reddish-brown |
| T. horrida | 17–36 | Polygonal to cerebriform scales | Sharply pointed to truncate | Chestnut brown |
| T. ehrhartae | 18–25 | Coarse polygonal scales | Cylindrical, truncate | Olivaceous, dark |
| T. rugispora | 17–28 | Coarse polygonal scales | Conical, bluntly pointed | Mid-reddish brown |
| T. barclayana | 14–36 | Fine, polygonal to cerebriform scales | Sharply pointed to truncate | Chestnut brown |
| S. No. | Assembly Name | GenBank Assembly ID | Submitter (Year) | Reference |
|---|---|---|---|---|
| 1. | AAFC_TiDAOMC236408_1 | GCA_009428345.1 | Agriculture and Agri-Food, Canada (2019) | [69] |
| 2. | AAFC_TiDAOMC236414_1 | GCA_009428365.1 | ||
| 3. | AAFC_TiDAOMC236416_2 | GCA_001645015.2 | ||
| 4. | ASM222083 v1 | GCA_002220835.1 | ICAR-IARI, India (2019) | [63] |
| 5. | ASM299730 v1 | GCA_002997305.1 | GBPUAT, India (2017 and 2018) | [67] |
| 6. | ASM305493 v1 | GCA_003054935.1 | [68] | |
| 7. | PSWKBGD_1_1 | GCA_001689995.1 | ICAR-IIWBR, India (2016) | [66] |
| 8. | PSWKBGD_1_2 | GCA_001689945.1 | ||
| 9. | PSWKBGD_1_3 | GCA_001689965.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iquebal, M.A.; Mishra, P.; Maurya, R.; Jaiswal, S.; Rai, A.; Kumar, D. Centenary of Soil and Air Borne Wheat Karnal Bunt Disease Research: A Review. Biology 2021, 10, 1152. https://doi.org/10.3390/biology10111152
Iquebal MA, Mishra P, Maurya R, Jaiswal S, Rai A, Kumar D. Centenary of Soil and Air Borne Wheat Karnal Bunt Disease Research: A Review. Biology. 2021; 10(11):1152. https://doi.org/10.3390/biology10111152
Chicago/Turabian StyleIquebal, Mir Asif, Pallavi Mishra, Ranjeet Maurya, Sarika Jaiswal, Anil Rai, and Dinesh Kumar. 2021. "Centenary of Soil and Air Borne Wheat Karnal Bunt Disease Research: A Review" Biology 10, no. 11: 1152. https://doi.org/10.3390/biology10111152